Controlled Drug Register Template
Controlled Drugs (CD) Register requirements are defined in UK law by the Medicines Act. We have created a CD register template to use free of charge based around this guidance, a picture of which can be seen below:
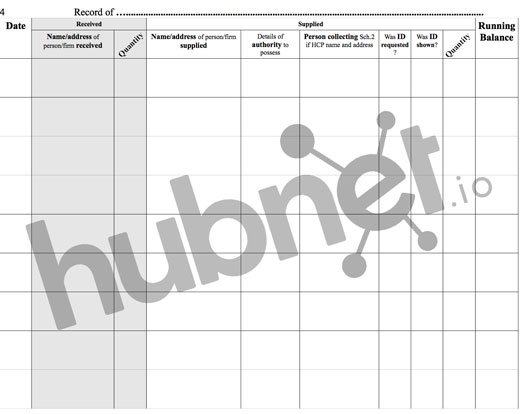
We have tried and tested this in our pilot pharmacy; you can download the PDF using the link below.
To purchase a low cost, 300-page copy, of a fully compliant CD register just click the links below:
- Methadone 1mg/ml - purchase from Lulu.com for £9+VAT!
- Methadone 10mg/ml - purchase from Lulu.com for £9+VAT!
Or use our unlimited entry:
Why use our Controlled Drugs Registers?
As you will see, all our registers meet the requirements set out in the Medicines Act, including the recording of:
(i) Date supply received;
(ii) name and address from whom received;
(iii) Quantity received.
(e) The headings in respect of entries made for drugs supplied are—
(i) Date supplied;
(ii) Name/Address of person or firm supplied;
(iii) Details of authority to possess— prescriber or licence holder’s details;
(iv) Quantity supplied;
(v) Person collecting Schedule 2 controlled drug (patient/patient’s rep/healthcare professional) and if healthcare professional, name and address;
(vi) Was proof of identity requested of patient/patient’s rep (Yes/No);
(vii) Was proof of identity of the person collecting provided (Yes/No).
Hubnet is an online pharmacy information system. We intend to provide healthcare professionals with an online ecosystem to allow for better communication between each other and their patients. Protected by law, the data you enter into this site remains your intellectual property and cannot be used by us. Our goal is to enable you to do more, if you like it you can subscribe for more!
